Pills To Treat COVID-19 Are Here… Sort Of
Author: Andrea Taylor
We are excited to release data on purchases of therapeutics to treat COVID-19. The latest numbers are available from the Duke Global Health Innovation Center at the Launch and Scale Speedometer project here.
Oral therapeutics for COVID-19 have been heralded as a game changer, with the first two recently coming to market from Pfizer (Paxlovid) and Merck (molnupiravir). For the past year, vaccines and social distancing measures have been our only significant tools to combat COVID19. As we try to prepare for whatever comes after the omicron variant, which caused infection and hospitalization rates to surge globally, oral therapeutics provide one more line of defense, particularly for those at most risk of bad outcomes.
It is important to note that the two oral therapeutics on the market differ significantly. Among people at high risk of severe illness or death from COVID-19, Merck’s molnupiravir demonstrated 30% efficacy at preventing severe disease, while Pfizer’s Paxlovid was 89% effective. The risks (and recommended populations) are different. Both have received conditional approval in the US and many other countries worldwide, though some countries have expressed reservations about widespread use of Merck’s product.
It is going to be a long wait
As promising as they are, these drugs are not likely to change the game for most of the world in 2022 for three reasons. First, the supply for most of this year will be very limited and nowhere near enough to meet the need. Second, much like we saw with vaccines in 2021, the initial supply from Pfizer and Merck is already locked up by wealthy countries. And finally, effective use of these tablets depends on robust diagnostic capacity and a strong test-and-treat strategy, which is not yet in place for most of the world’s countries.
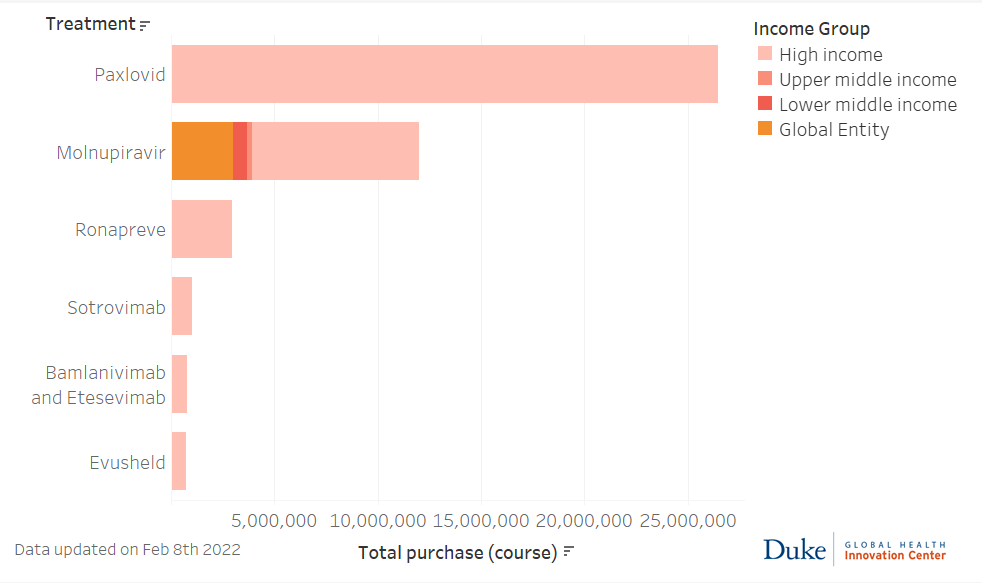
Source: Launch and Scale Speedometer, Duke Global Health Innovation Center. Updated February 4, 2022
There is good news
The makers of the oral therapeutics that have made it to market so far do appear to have learned from the disastrously inequitable distribution of vaccines in 2021. Both Pfizer and Merck have partnered with the Medicines Patent Pool (MPP) to allow generic manufacturing of their COVID-19 treatments. The MPP announced last month that 27 manufacturers in 11 countries have signed agreements to produce molnupiravir. A similar announcement for generic manufacturing of Paxlovid is expected in early March.
This will (eventually) support global access by allowing the pills to be manufactured in and distributed by low- and middle-income countries (LMICs). However, it looks like it will take between 9 and 12 months before the generic manufacturers licensed through MPP begin manufacturing at scale. While fast tracking generic manufacturing for LMIC distribution through the MPP is a welcome development, it is likely to pay dividends in 2023 rather than this year.
Counterfeit concerns
In the meantime, there are serious regulatory and quality concerns with generic versions of these products making it onto shelves in LMICs now. Ads on social media boast availability of generic molnupiravir and Paxlovid in many LMICs, including Thailand, Vietnam, and Myanmar, through private sellers and health clinics. And just a week after approving the oral therapeutics from Pfizer and Merck, the Mexican government warned of counterfeit versions for sale, manufactured by companies without regulatory or import approval.
With demand likely to outstrip supply for 2022, there is a high risk of black-market sales and fake drugs. For countries with low vaccination rates, the self-administered tablets may offer the only available protection against severe disease, increasing vulnerability to fakes.
We must prepare now
We now know that oral therapeutics can effectively treat COVID-19, providing another option to protect those most at risk of serious illness or death. We are not likely to see sufficient supply until 2023 but we can use the lead time strategically to ensure the world is ready when supply is available.
This must include a renewed focus on (and funding for) diagnostic manufacturing and distribution, as well as the development of test and treat strategies that can be rolled out at the country level. The oral therapeutics are most effective if taken within a few days of infection, meaning that people at risk of severe illness need to know they are infected and have almost immediate access to the drug after a positive test.
In addition, we must develop regulatory and distribution pathways that ensure high quality products will be available everywhere. Given the unpredictable nature of this virus, we should plan for global stockpiles that can address surges when and where they occur.
We will continue to track purchases, manufacturing, and the pipeline of COVID-19 therapeutics over the coming months. Through our work in the COVID GAP initiative, we are also looking closely at the potential role of COVID-19 therapeutics and recommendations for a global roll out, and we expect to publish a report on this topic by the end of February.
As always, please email us at info@launchandscalefaster.org if you have any additional data points or corrections for our COVID-19 therapeutics or vaccine data. We would love to hear about how you are using the data and are grateful for your support in making our data as complete and accurate as possible.
