Authors: Katharine Olson and Wenhui Mao
Vaccines have played a large role in the global COVID-19 response and saved numerous lives. However, when highly transmissible variants such as Omicron (BA.1) appeared and quickly dominated the reported cases, the effectiveness of vaccinations was noticeably waning. The first U.S. case of BA.1 was reported December 1st, 2021, and by January 8th, 2022 it comprised 95% of new cases. In the last week of January 2022, confirmed new cases worldwide hit 23 million which is in stark contrast to previous highs of 5 million new cases a week. At the start of the BA.1 wave in late 2021, the primary vaccination series was only 44% effective at preventing infection and hospitalization. This prompted the push globally to administer booster vaccine doses as three doses were found to be 71.6% effective against BA.1. Waning effectiveness led vaccine manufacturers to create bivalent vaccines that could target two COVID-19 strains and provide better protection.
In this blog, we describe the features of the available bivalent COVID-19 vaccines, track the regulatory approval and the procurement of bivalent COVID-19 vaccines, new vaccine technologies in clinical development, and implications of introducing these medical countermeasures.
Mechanism and efficacy of the bivalent COVID-19 vaccines
Bivalent vaccines are one of the newest tools to protect against COVID-19 infection and severe outcomes. Bivalent COVID-19 vaccines contain a portion of the spike protein from the original wild-type strain as well as a portion of the omicron spike protein. Both Moderna (Spikevax) and Pfizer (Comirnaty) have updated their existing monovalent mRNA vaccines to include an omicron component, whether that be a portion of the original BA.1 or subvariant BA.4/5 spike protein.
Early clinical trials indicated that administration of a bivalent booster dose increased the average titer of neutralizing antibodies to BA.1 by 1.6 times that of a monovalent booster in those with no prior COVID-19 infection. In addition, administration of a bivalent booster dose increased neutralizing antibodies to BA.4/5 by 1.5 times that of a monovalent booster. While statistically significant differences in antibody titers have been found, there is concern that this might not translate to clinical significance.
Bivalent vaccines are meant to be administered as booster doses after the completion of the primary vaccination series and are recommended to be prioritized for high-risk populations. Similar to how the influenza vaccines work, the idea is that as the circulating COVID-19 strain changes, the boosters can be updated to provide targeted protection. These bivalent COVID-19 vaccines come in advance of a predicted surge in cases this fall and winter in the northern hemisphere, providing an extra line of defense for those who need it most.
Regulatory approval and procurement of bivalent COVID-19 vaccines
Currently, the bivalent vaccines from Pfizer and Moderna have received provisional approval in only a handful of high-income countries (HICs) or regions: European Commission, Italy, USA, UK, Israel, Canada, Australia, Taiwan, South Korea, and Japan. While the BA.4/5 subvariant has replaced BA.1 as the widely circulating strain, several countries have opted to prioritize purchasing vaccines that contain the BA.1 protein. Only the United States and Israel have purchased BA.4/5 bivalent vaccines. The discontinuity in approval of these vaccines globally coupled with the lack of clear clinical efficacy may create “two tiers” of vaccines, making it harder to achieve high levels of booster coverage and fueling vaccine hesitancy.
Purchases of these bivalent COVID-19 vaccines have come from COVAX and high-income entities which have achieved high levels of primary vaccination coverage, and are now focused on getting booster shots to vulnerable populations. There is significant vaccine supply available and wealthy countries have purchased far more doses than needed to vaccinate priority populations. Australia has purchased 15 million bivalent doses, enough to vaccinate the age 65+ population 3.5 times and the healthcare worker population 8.4 times. The United States has purchased nearly 171 million doses of bivalent vaccines which is over 3 times as many doses needed to vaccinate its 65+ and healthcare worker populations. In contrast to WHO recommendations that booster doses should be prioritized amongst high-risk populations, the United States is encouraging all age groups to take the bivalent vaccine, which may in part explain the disparity in doses purchased. Canada has purchased 12 million doses, which could vaccinate the 65+ population 1.7 times and the healthcare worker population 4.6 times.
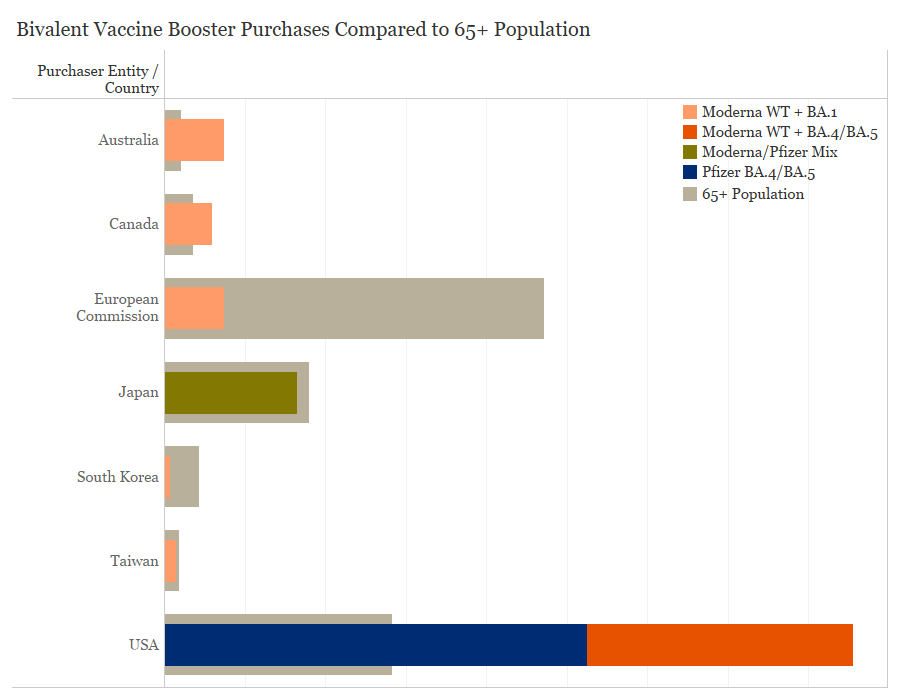
Purchases of bivalent COVID-19 vaccines by country and population of healthcare workers, Data collected by the Duke Global Health Innovation Center Launch and Scale Speedometer research team; healthcare worker and 65+ population data from WHO CRD and World Bank
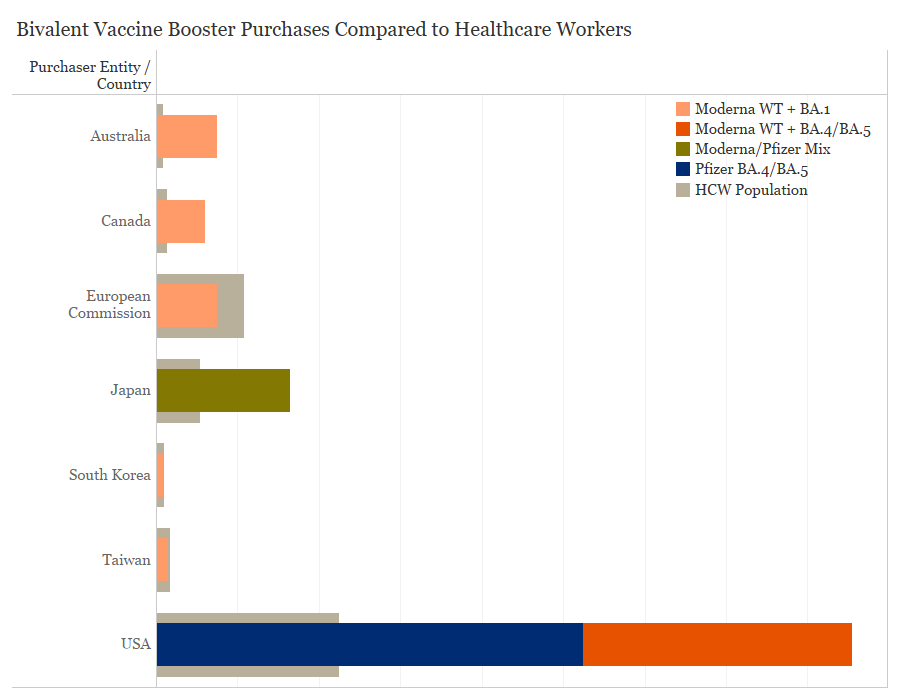
Purchases of bivalent COVID-19 vaccines by country and population over the age of 65, Data collected by the Duke Global Health Innovation Center Launch and Scale Speedometer research team; healthcare worker and 65+ population data from WHO CRD and World Bank
Upcoming COVID-19 products in the clinical development pipeline
The bivalent vaccines are one of many potential new tools in the COVID-19 response. Several new vaccine candidates are in clinical development or have recently been approved. Intradermal “patch” vaccines are shown to be stable without refrigeration and could be self-administered, potentially solving cold chain and health workforce vaccination training barriers. Additionally, inhaled COVID-19 vaccines have been approved such as CanSino Biologics’ vaccine in China and Bharat Biotech’s vaccine in India. These are just two of sixteen oral and nasal vaccine candidates that have reached clinical trials in humans. Oral and nasal vaccines are thought to provide an advantage over conventional vaccines because they target the immune cells in the nose and mouth which are the first line of defense against COVID-19 infection. The hope is that this will prevent mild cases by stopping the virus at its point of entry while potentially blocking transmission to other people. Vaccines that can block transmission of COVID-19 would help protect those who cannot be vaccinated and limit the development of new variants that drive the ongoing pandemic.
|
Route of vaccine administration |
Number in Clinical Development |
% (of 172 total) |
|
Injectable |
156 |
91% |
|
SC Sub cutaneous |
5 |
3% |
|
ID Intra dermal |
9 |
5% |
|
IM Intra muscular |
142 |
83% |
|
IN Intra nasal |
13 |
8% |
|
AE Aerosol |
1 |
1% |
|
IH Inhaled |
2 |
1% |
|
TBD/No Data (ND) |
11 |
6% |
Breakdown of the 172 COVID-19 vaccine candidates in clinical development by route of administration, Source: WHO COVID-19 Vaccine Tracker
Moving forward
Even though the regulatory approval and procurement of bivalent vaccines has only happened among HICs, there are some positive developments. The United Kingdom has already donated doses of the Moderna bivalent vaccine, and Gavi has signed an agreement with Moderna to provide access to up to 100 million bivalent vaccine doses via the COVAX platform.
However, there are many hurdles before the bivalent COVID-19 vaccines can truly contribute to the pandemic control globally. Bivalent vaccines have only been approved for use as boosters not for the primary vaccination series. Primary vaccination coverage is 19.5% of the total population in low-income countries, and booster coverage is even lower. Countries and manufacturers alike have had to destroy expired monovalent vaccines because supply far outweighs demand. Without proper coordination and sufficient clinical evidence, pushing to use these new tools may result in vaccine wastage in countries whose priorities remain on increasing primary COVID-19 vaccination.
There are hesitations around whether bivalent vaccines are effectively any better at preventing infection than current vaccines on the market, and whether BA.4/5 will be the primary strain circulating in the fall. The hope for bivalent vaccines is that they will pave the way for development of multivalent or pan-coronavirus vaccines that would provide broader protection than existing vaccines. Additionally in the United States alone, demand for bivalent boosters is modest with roughly 30% of adults saying they plan to get the booster as soon as possible. Considering the quantity of doses purchased by the U.S., low demand for vaccinations could lead to wastage.
As bivalent vaccines saturate HIC markets and new vaccine technologies are approved, it is imperative that regulatory and distribution pathways are created and maintained to ensure that these products are available everywhere. This is especially important as major funding mechanisms, such as ACT-A, are transitioning from emergency response to long-term disease control. Now more than ever, there must be renewed effort to enhance low and middle-income countries’ vaccination strategies and ensure equitable access to new vaccine technologies.
We are excited to release data on purchases of bivalent COVID-19 vaccines. The latest numbers are available from the Duke Global Health Innovation Center at the Launch and Scale Speedometer project here.
