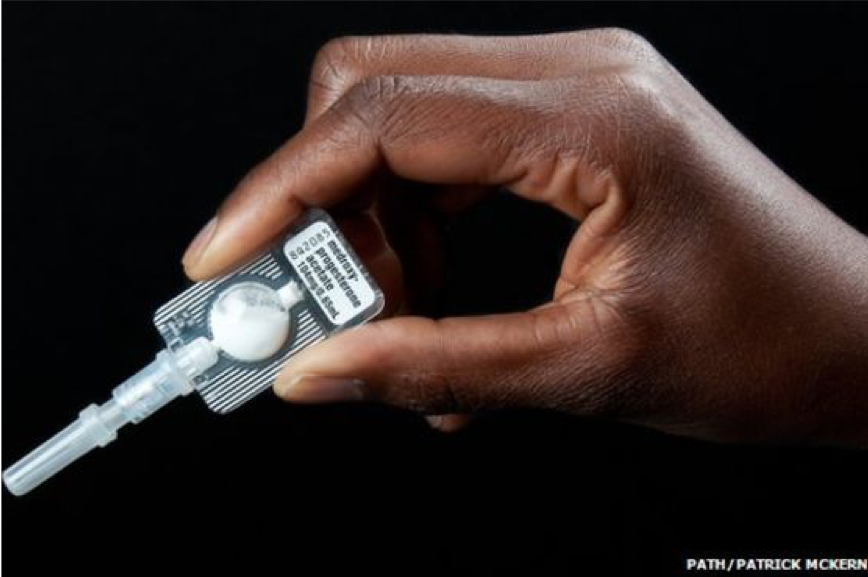
An easy-to-administer three-month injectable contraceptive (depot-medroxyprogesterone acetate (DMPA-SC)) administered into the fat below the skin.
In low- and middle-income countries (LMICs), it is estimated that 190 million women have an unmet need for contraception despite a wide range of contraceptive options available in many markets. Injectable contraceptives are one method of contraception and Pfizer’s Sayana® Press, the only available DMPA-SC product on the market, is a small, all-in-one injection system that prevents pregnancy for three months. Since Sayana® Press is so easy to use it does not require much if any training, so it can easily be incorporated into existing service channels. This also makes it suitable for self-injection, which further decreases barriers to access, and in some markets Sayana® Press is labelled and sold for self-injection.
The political drive to provide millions of women in LMICs with access to contraception in response to the large unmet need has played a central role in supporting Sayana® Press launch into markets. In 2012, the Family Planning 2020 (or FP2020) public-private partnership emerged to provide billions in funding to support progress towards the goal of providing 120 million additional women with access to contraceptives in 69 countries. In 2017 the Access Collaborative, working to accelerate the scale up of Sayana® Press, negotiated a lower price for qualified buyers at US$0.85 per dose, which makes it competitive with intramuscular forms of DMPA. In 2019 Sayana® Press was available in more than 30 FP2020 countries.
